The Heart Group of Syracuse is pleased to offer the S-ICD™ System, the first non-invasive Subcutaneous ICD implantation. The Heart Group of Syracuse is the first cardiology group in Upstate New York to offer this revolutionary non-invasive procedure.
What is the S-ICD™ System?
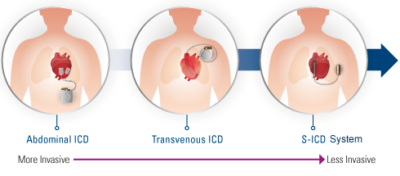
For years, implantable cardioverter defibrillators, or transvenous ICDs (TV-ICDs) have saved the lives of patients at risk of sudden cardiac arrest. Today, a new exciting therapeutic solution for patients is available: the S-ICD™ System, which provides patients protection from sudden cardiac arrest without touching the heart.
Protection Without Touching the Heart
The S-ICD System is the world’s first and only subcutaneous implantable defibrillator that provides protection from sudden cardiac arrest (SCA) while leaving the heart and vasculature untouched. Like transvenous ICDs, the S-ICD System utilizes a pulse generator capable of delivering life-saving therapy. Unlike transvenous ICDs, the S-ICD System uses a subcutaneous electrode and analyzes the heart rhythm – rather than individual beats – to effectively sense, discriminate, and convert VT/VF. For years patients’ lives have been extended by implanting transvenous implantable defibrillators. Now the S-ICD System provides a new solution to protect patients from SCA, without touching the heart.
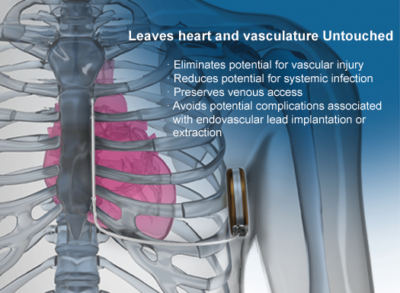
Effective Defibrillation Without Transvenous Leads
- Effective detection and conversion of induced and spontaneous VT/VF episodes1,2
- Low rates of significant clinical complications1
- Effective discrimination of AF and SVT from VT/VF1,2,3
- Rate of inappropriate therapy is consistent with transvenous ICDs1,2,3
Sophisticated Technology
- Identifies and classifies the heart rhythm, rather than individual beats
- Revolutionary approach to sensing the subcutaneous signal
- INSIGHT™ algorithm effectively discriminates between treatable and other high-rate supra-ventricular events
- Designed to allow self-termination of non-sustained tachyarrhythmia
Durable Subcutaneous Electrode Design
- 45cm length, which includes two sensing electrodes and one shocking coil
- Subcutaneous placement avoids intra-cardiac biomechanical stresses
- Multistrand cable-core design provides exceptional tensile strength
- Durable polyurethane body is highly resistant to abrasion
New Solution for a Broad Range of Patients at Risk for SCA
- Effective for a majority of primary and secondary ICD candidates*
- Alternative for TV-ICD replacements due to lead malfunction/infection
- Ideal for primary electrical or structural heart disease
- Appropriate for a broad range of body types

